Abstract
Introduction: With a median age of diagnosis in the late 60s, many patients with Acute Myeloid Leukemia (AML) are deemed unsuitable for conventional curative therapy (typically anthracycline and ara-C). The decision may be based upon a patient's age, cytogenetic profile, fitness, comorbidities, or indeed a preference for less-intensive therapy. Survival in these patients has traditionally been poor with <20% typically surviving at 2 years, whether treated with regimens such as low-dose ara-C (LDAC) or hypomethylating agents. There is a clear unmet clinical need in these patients both to improve survival and also to maximise quality of life (QoL) in that period. The UK NCRI LI-1 trial has evaluated a number of novel agents, either as monotherapy or in combination with LDAC. While some agents have improved remission rates, none has so far demonstrated improved survival. However, achievement of remission may itself represent an improvement for the patient. We therefore evaluated the impact of remission on patient QoL.
Methods: Within the LI-1 trial, QoL using EORTC, QLQ30C and EQ-5D3L was collected at baseline, 3, 6 and 12 months. Summary scores were calculated according to the scoring manuals for these instruments with QLQ30C summarised using the summary score obtained from the 13 individual dimensions. QLQ30C and EQ5D Visual Analogue Score (VAS) were scored from 0-100; the EQ5D utility score had a maximum of 1 (equating to perfect health). For the purposes of this analysis data from all trial arms were combined. Comparisons at individual timepoints were performed using Student's t-test, with Mixed Models Repeated Measures analysis performed to give overall differences across timepoints.
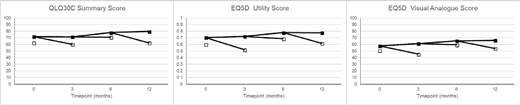
Results: A total of 1653 questionnaires (baseline n=827; 3 month n=402; 6 month n=280, 12 month n=143) were received from 833 patients. On all measures, QoL was lower in patients not surviving to the next assessment point (Figure); using repeated measures analysis, patients who died before the next QoL assessment point had QLQ30C summary score 8.65 (6.68-10.61) points lower, EQ5D utility score 0.11 (0.08-0.14) points lower, and EQ5D VAS 7.73 (5.13-10.34) lower (all p<.0001). After early deaths had been excluded, there were no significant differences in baseline quality of life between those patients who entered remission and those who did not on any measure. In an analysis where patients who died within 90 days of the assessment were excluded to allow for the effect of impending mortality on QoL, patients who were in remission at a post-baseline timepoint had significantly improved QLQ30C summary score (difference 4.27 (0.06-8.48), p=0.05), and EQ5D utility score (difference 0.08 (0.02-0.14) p=0.008), but EQ5D VAS was not significantly different by remission status (-1.32 (-7.57-4.93) p=0.7). When individual timepoints were considered, point estimates favoured remission for QLQ30C summary score, and EQ5D utility score at all time points, reaching significance at 3 months on both scores, and at 6 months on the EQ5D utility score.
Conclusion: This is the largest study to date of quality of life in this population. Patients who died demonstrated a downturn in QoL, measured either using QLQ30C, EQ5D Utility Score or EQ5D VAS at the assessment immediately prior to their death. In particular, early mortality was associated with lower QoL at baseline, although there was considerable overlap meaning it is unlikely that QoL alone can be used to guide treatment. Additionally, in patients achieving complete remission, both the QLQ30C and EQ5D Utility Score were significantly higher at timepoints when the patient was in remission, although differences were modest. The EQ5D VAS was not however significantly different. These data indicate that even without prolonging survival, an increase in complete remission rates may be associated with patient benefit. Clinical trials in this population should include QoL measures as a matter of course, as treatments which improve remission rates may deliver meaningful benefit in terms of QoL and health utility. Further work is required to determine whether, in light of the lack of benefit on the patient's own summary measure of their QoL, these potential improvements are perceived by patients as being worthwhile.
Figure caption: Quality of Life scores at each timepoint - open boxes represent patients who die before the next assessment is due (or 18 months in the case of the 12 month assessment).
Hills:Daiichi Sankyo: Consultancy, Honoraria. Russell:Jazz Pharma: Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau; Daiichi Sankyo: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal